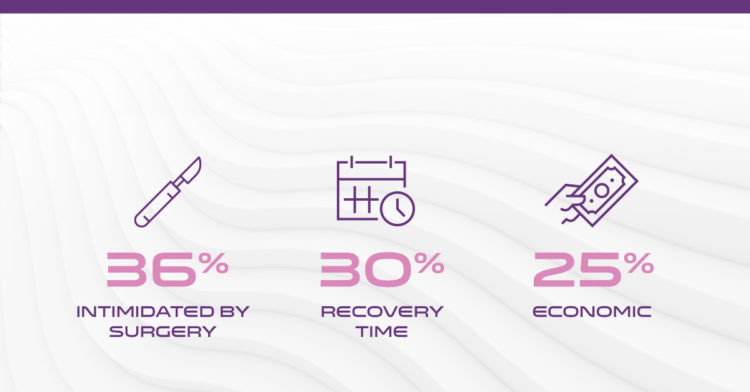
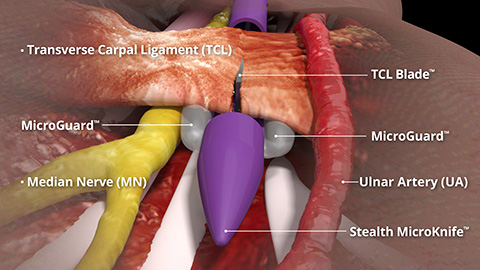
4 Out of 5 patients indicated for CTR avoid or delay surgery
Carpal Tunnel Syndrome
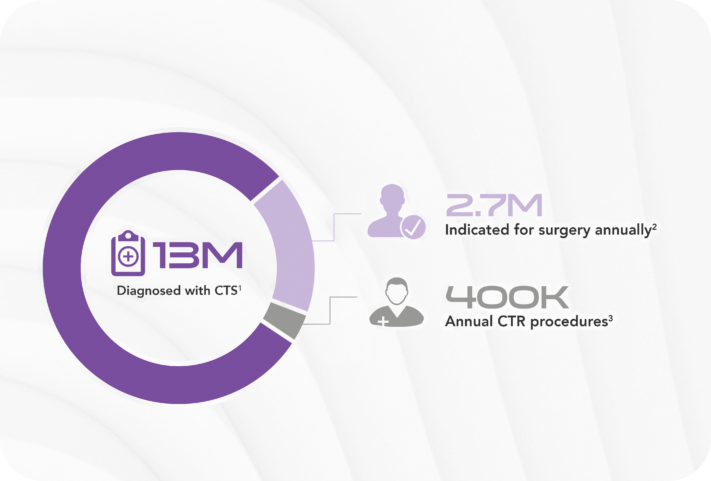
Carpal tunnel syndrome (CTS) affects an estimated 13 million1 Americans causing pain and keeping people from work and the activities they love. More than 2.7 million2 are clinically indicated for surgery, yet there are only approximately 400,0003 procedures performed every year.
*Image Reference: 4 out of 5 patients indicated for CTR avoid or delay surgery.1-3